› Forums › General Melanoma Community › For all you stage IV patients, a collection of response rates by the Therapy
- This topic has 40 replies, 7 voices, and was last updated 14 years, 7 months ago by
MariaH.
- Post
-
- July 20, 2011 at 11:09 pm
Patients with metastatic melanoma have a poor prognosis with a 5 year survival rate of about 5%1. There are two FDA approved treatments for these patients.
Patients with metastatic melanoma have a poor prognosis with a 5 year survival rate of about 5%1. There are two FDA approved treatments for these patients.
Dacarbazine has an objective response rate of approximately 12% with 2-3 % complete responses that are often transient2.
Interleukin-2 (IL-2) has an objective response rate of approximately 15% with 4-5% durable complete responses3.
Results of two new experimental agents have recently been reported for the treatment of patients with this disease.
Ipilimumab, an antibody against the inhibitory lymphocyte receptor, CTLA4, mediated a 3.6 month improvement in median survival with an objective response rate of 7% in 540 patients but only three patients (0.6%) achieved a complete regression4.
PLX4032, an inhibitor of mutated BRAF, had an objective response rate of 77% in 48 patients with 3 (6%) complete regressions5.
The very small number of durable complete responses make it unlikely that many patients with metastatic melanoma will be cured utilizing any of these approaches.
This is why we must try combinatorial Therapy with or without vaccines or Adoptive cell therapy (TIL) with Dr. Rosenberg.
IMPACT OF PRIOR TREATMENT ON RESPONSE TO CELL TRANSFER THERAPY USING SELECTED TIL
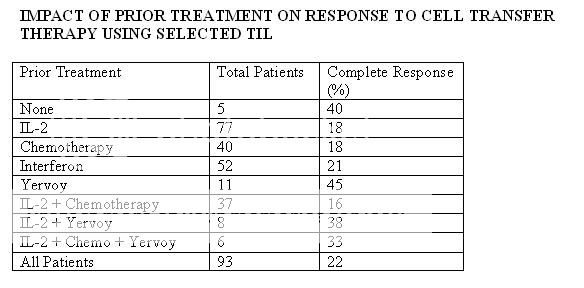
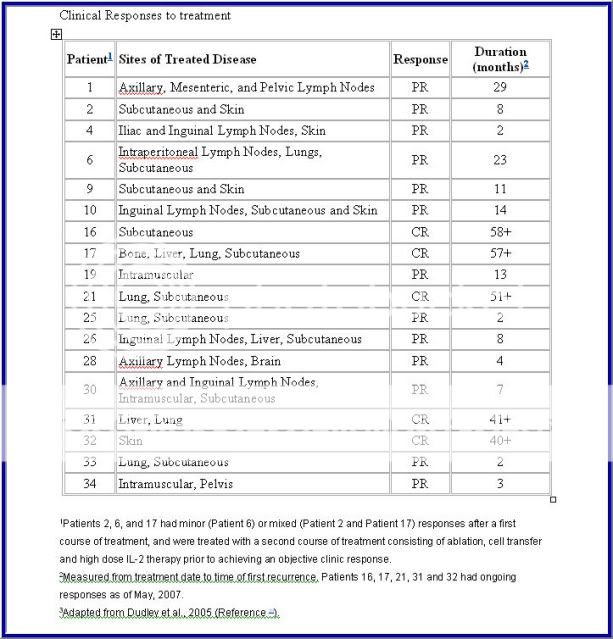
Source: Durable Complete Responses in Heavily Pretreted Patients with Metastatic Melanoma Using T Cell Transfer Immunotherapy
Cancer Res Published OnlineFirst April 15, 2011.
Steven A. Rosenberg, James C. Yang, Richard M. Sherry, et al.
=========================================================================
Based on 2005 data… Rosenberg has improved the protocol since then.
I thought you might like to know
- Replies
-
-
- July 21, 2011 at 12:25 am
Meeting:
Session Type and Session Title:
General Poster Session, Melanoma/Skin Cancers
Abstract No:
8544
Citation:
J Clin Oncol 28:15s, 2010 (suppl; abstr 8544)
Author(s):
P. A. Prieto, J. C. Yang, R. M. Sherry, M. S. Hughes, U. S. Kammula, D. E. White, C. L. Levy, S. A. Rosenberg, G. Q. Phan; Surgery Branch, National Cancer Institute, National Institutes of Health, Bethesda, MD; National Cancer Institute, National Institutes of Health, Bethesda, MD; National Cancer Institute, Bethesda, MD; Surgery Branch, National Cancer Institute, Bethesda, MD
Abstract:
Background: We have previously shown objective clinical responses in patients with metastatic melanoma treated with CTLA-4 blockade using ipilimumab. We have treated 179 patients in 3 separate clinical trials and now have long-term follow-up to evaluate the durability and unique features of this immunotherapy.Methods: A total of 179 patients with metastatic melanoma were treated in 3 trials: In Protocol 1, 56 patients received ipilimumab with gp100 peptide vaccines. In Protocol 2, 36 patients received ipilimumab with high-dose interleukin-2 (IL-2). In Protocol 3, 87 patients received intra-patient dose escalation of ipilimumab and were randomized to receive gp100 peptides. We have updated and analyzed the follow-up and survival data for these trials.Results: With median follow-up for Protocol 1, 2, and 3 being 80, 71, and 60 months, median survival was 15, 16, and 13 months, respectively. Objective tumor regression was 12% for Protocol 1, 25% for Protocol 2, and 21% for Protocol 3. Patients in Protocol 2 had a 17% complete response rate (6 patients: 77+, 74+, 72+, 71+, 71+, and 69+ months), as compared to 7% in Protocol 1 (4 patients: 82+, 81+, 79+, and 66+ months) and 8% in Protocol 3 (5 patients: 64+, 63+, 62+, 60+, and 55+ months); all complete responses are ongoing. Many patients who eventually became complete responders had continual tumor shrinkage after stopping therapy.Conclusions: CTLA-4 blockade with ipilimumab can achieve durable objective tumor regression in patients with metastatic melanoma. The combination of ipilimumab and IL-2 appears to have an increased complete response rate, although this needs to be tested in a prospective randomized trial. This report represents the largest single-institution experience with the longest follow-up for this agent; our results support its role as a viable treatment option for patients with metastatic melanoma.
As a Melanoma Stage IV survivor that benefited by the above combination with a complete response.
I was wondering if any progress has been made in intoducing a clinical Trial Protocol with Yervoy and IL-2?
So I contacted some of the major Oncologists that deal with these therapies.
THE ANSWER …. IS ASTOUNDING NO!!!
The time is NOW to get engaged and focus

Focus on a COMPLETE RESPONSE >> A CURE
-
- July 21, 2011 at 12:25 am
Meeting:
Session Type and Session Title:
General Poster Session, Melanoma/Skin Cancers
Abstract No:
8544
Citation:
J Clin Oncol 28:15s, 2010 (suppl; abstr 8544)
Author(s):
P. A. Prieto, J. C. Yang, R. M. Sherry, M. S. Hughes, U. S. Kammula, D. E. White, C. L. Levy, S. A. Rosenberg, G. Q. Phan; Surgery Branch, National Cancer Institute, National Institutes of Health, Bethesda, MD; National Cancer Institute, National Institutes of Health, Bethesda, MD; National Cancer Institute, Bethesda, MD; Surgery Branch, National Cancer Institute, Bethesda, MD
Abstract:
Background: We have previously shown objective clinical responses in patients with metastatic melanoma treated with CTLA-4 blockade using ipilimumab. We have treated 179 patients in 3 separate clinical trials and now have long-term follow-up to evaluate the durability and unique features of this immunotherapy.Methods: A total of 179 patients with metastatic melanoma were treated in 3 trials: In Protocol 1, 56 patients received ipilimumab with gp100 peptide vaccines. In Protocol 2, 36 patients received ipilimumab with high-dose interleukin-2 (IL-2). In Protocol 3, 87 patients received intra-patient dose escalation of ipilimumab and were randomized to receive gp100 peptides. We have updated and analyzed the follow-up and survival data for these trials.Results: With median follow-up for Protocol 1, 2, and 3 being 80, 71, and 60 months, median survival was 15, 16, and 13 months, respectively. Objective tumor regression was 12% for Protocol 1, 25% for Protocol 2, and 21% for Protocol 3. Patients in Protocol 2 had a 17% complete response rate (6 patients: 77+, 74+, 72+, 71+, 71+, and 69+ months), as compared to 7% in Protocol 1 (4 patients: 82+, 81+, 79+, and 66+ months) and 8% in Protocol 3 (5 patients: 64+, 63+, 62+, 60+, and 55+ months); all complete responses are ongoing. Many patients who eventually became complete responders had continual tumor shrinkage after stopping therapy.Conclusions: CTLA-4 blockade with ipilimumab can achieve durable objective tumor regression in patients with metastatic melanoma. The combination of ipilimumab and IL-2 appears to have an increased complete response rate, although this needs to be tested in a prospective randomized trial. This report represents the largest single-institution experience with the longest follow-up for this agent; our results support its role as a viable treatment option for patients with metastatic melanoma.
As a Melanoma Stage IV survivor that benefited by the above combination with a complete response.
I was wondering if any progress has been made in intoducing a clinical Trial Protocol with Yervoy and IL-2?
So I contacted some of the major Oncologists that deal with these therapies.
THE ANSWER …. IS ASTOUNDING NO!!!
The time is NOW to get engaged and focus

Focus on a COMPLETE RESPONSE >> A CURE
-
- July 21, 2011 at 12:31 am
Hi Jimmy,
Being Stage 4 myself, I don't like these stat numbers and have been told by my own Dr a different story. 5 year survival stats for Stage 4 are upwards of 20% now because of new treatments. I prefer to think I have a 20% chance rather than a 5%, but also know alot of people beat the odds and I'll be one of them too.
Dacarbazine has a 15-20% response of working and seems to show more activity in low tumour burden. ipi (Yervoy) I was told is 25-30% response and patients who have complete response do so for upwards of 3 years. These "stats" are more encouraging. Perhaps each Dr has different stats which is why many people seem to be given different numbers. Or, maybe the stats I've been given are Canadian stats.
Regardless, there is no cure for melanoma and stats are just stupid stats. Jimmy – let's hope they find the perfect combo (seems to be alot of talk about dacarbazine and ipi) and TIL will hopefully continue to show strong numbers so that it can one day be the standard treatment for Stage 4.
Lisa
-
- July 21, 2011 at 12:31 am
Hi Jimmy,
Being Stage 4 myself, I don't like these stat numbers and have been told by my own Dr a different story. 5 year survival stats for Stage 4 are upwards of 20% now because of new treatments. I prefer to think I have a 20% chance rather than a 5%, but also know alot of people beat the odds and I'll be one of them too.
Dacarbazine has a 15-20% response of working and seems to show more activity in low tumour burden. ipi (Yervoy) I was told is 25-30% response and patients who have complete response do so for upwards of 3 years. These "stats" are more encouraging. Perhaps each Dr has different stats which is why many people seem to be given different numbers. Or, maybe the stats I've been given are Canadian stats.
Regardless, there is no cure for melanoma and stats are just stupid stats. Jimmy – let's hope they find the perfect combo (seems to be alot of talk about dacarbazine and ipi) and TIL will hopefully continue to show strong numbers so that it can one day be the standard treatment for Stage 4.
Lisa
-
- July 21, 2011 at 12:37 am
Lisa,
These stats came from a paper dated April 11 2011 written by Dr. Steven Rosenberg of the NCI. I believe they are from a good source.
Jimmy B
-
- July 21, 2011 at 12:37 am
Lisa,
These stats came from a paper dated April 11 2011 written by Dr. Steven Rosenberg of the NCI. I believe they are from a good source.
Jimmy B
-
- July 21, 2011 at 4:06 am
I keep thinking they intentionally leave surgery and radiation out of the picture and yet surgery is a preferred initial treatment for many people…Stage 4 NED since March 26, 2010. ..and had radiation, temodar, surgery and vaccine trial of peptides and anti pd 1. Been NED since surgery.
-
- July 21, 2011 at 12:48 pm
Lynn,Congrats!!!
Been NED since surgery!!!!!
Why did you do radiation, temodar, surgery and vaccine trial of peptides and anti pd 1. since surgery? Do them as Adjuvant Therapy? You must have found all the trials that would accept NED patients. Please explain.
Warm regards
Jimmy B
-
- July 23, 2011 at 1:44 am
I have been NED since surgery when they took out the 6.8 melanoma from my lymph node.
I had radiation and temodar first to shrink it away from my superior vena cava because it was cutting off my blood supply to the upper have of my body.Mayo felt the Temodar would make melanoma more reactive to the radiation so after the first week of radiation I was put on Temodar….had the second week of radiation.
They knew the Temodar had a life of about 5-7 months before melanoma would be back…which became obvious in Feb 2010 when the SUV jumped from 4 point something to over 7.
The surgeon who did the biopsy wouldn't remove it at the time because the biopsy was done thru a tiny incision on the front of my neck with a Storz video. He saw in my right paratracheal lymph node region a very large black lymph node….he said it was a very dangerous place to operate. he only got little bites of it for biopsy.That was done on July 18, 2009.
I had planned to get into a trial in Seattle and I did go…they took my T cells and cloned them for 4 months ( they are currently frozen down until I need them they said) but after I go back from Seattle in Feb 2010 my PET showed that jump…so I went back to Mayo and my surgeon had agreed and did the thoracotomy,,,,dang that was an awful awful surgery. He removed a melanoma from the lymph node and it was 6.8 at the biggest part.
But I was surgically made NED….Seattle said I no longer qualified for their trial because I had no measurable disease.
No trial was available for people NED…
Then a miracle happened and Dr Weber had one with MDX 1106 and peptides. I did 12 weeks of MDX 1106 ( anti PD 1 ) and peptides and remain NED and I continue every 3 months to receive the booster of MDX 1106. I get the booster for at least 2 years. The reason we did the trial was every doctor including Dr. W said i had a 99% chance of it coming back…considering the size and the location from the original site…I was staged with Metastatic Melanoma stage 4.
Science behind is the less tumor burden ( or in my case NED) the better chance we have of keeping it down. Dr W says if I remain NED for the 2 years of boosters it would be in best interest to try and remain on it forever…makes sense to me! On July 26 it will be 16 months NED.
-
- July 23, 2011 at 1:50 am
PS I did TWO 12 week trials of the MDX 1106 and Peptides… but I went every other week equaling 12 IV's of MDX 1106 and 72 injections of peptides in my thighs ( those suckers hurt…they use horse needles and peptides are in a thick creamy base…They freeze the thigh with ice packs for at least a half hour before injecting them…
-
- July 23, 2011 at 1:50 am
PS I did TWO 12 week trials of the MDX 1106 and Peptides… but I went every other week equaling 12 IV's of MDX 1106 and 72 injections of peptides in my thighs ( those suckers hurt…they use horse needles and peptides are in a thick creamy base…They freeze the thigh with ice packs for at least a half hour before injecting them…
-
- July 23, 2011 at 1:50 am
PS I did TWO 12 week trials of the MDX 1106 and Peptides… but I went every other week equaling 12 IV's of MDX 1106 and 72 injections of peptides in my thighs ( those suckers hurt…they use horse needles and peptides are in a thick creamy base…They freeze the thigh with ice packs for at least a half hour before injecting them…
-
- July 23, 2011 at 1:59 am
They did not plan to do surgery at all…the plan was just to shrink it and kill it a little so my blood could keep flowing to my brain and heart etc etc.. radiation and temodar shrunk it by about a third…and it was stable not growing not spreading…I think the SUV remained ay 4.3 . It wasn't until it woke up and started to grow the surgeon agreed to do the surgery.
The only reason he did( dr Shen at Mayo )was because it hadn't spread…and because I wasn't dead :o). They gave me 6 to 9 months and so when I went back and said I was still here…what next???
-
- July 23, 2011 at 1:59 am
They did not plan to do surgery at all…the plan was just to shrink it and kill it a little so my blood could keep flowing to my brain and heart etc etc.. radiation and temodar shrunk it by about a third…and it was stable not growing not spreading…I think the SUV remained ay 4.3 . It wasn't until it woke up and started to grow the surgeon agreed to do the surgery.
The only reason he did( dr Shen at Mayo )was because it hadn't spread…and because I wasn't dead :o). They gave me 6 to 9 months and so when I went back and said I was still here…what next???
-
- July 23, 2011 at 1:50 am
PS I did TWO 12 week trials of the MDX 1106 and Peptides… but I went every other week equaling 12 IV's of MDX 1106 and 72 injections of peptides in my thighs ( those suckers hurt…they use horse needles and peptides are in a thick creamy base…They freeze the thigh with ice packs for at least a half hour before injecting them…
-
- July 23, 2011 at 1:44 am
I have been NED since surgery when they took out the 6.8 melanoma from my lymph node.
I had radiation and temodar first to shrink it away from my superior vena cava because it was cutting off my blood supply to the upper have of my body.Mayo felt the Temodar would make melanoma more reactive to the radiation so after the first week of radiation I was put on Temodar….had the second week of radiation.
They knew the Temodar had a life of about 5-7 months before melanoma would be back…which became obvious in Feb 2010 when the SUV jumped from 4 point something to over 7.
The surgeon who did the biopsy wouldn't remove it at the time because the biopsy was done thru a tiny incision on the front of my neck with a Storz video. He saw in my right paratracheal lymph node region a very large black lymph node….he said it was a very dangerous place to operate. he only got little bites of it for biopsy.That was done on July 18, 2009.
I had planned to get into a trial in Seattle and I did go…they took my T cells and cloned them for 4 months ( they are currently frozen down until I need them they said) but after I go back from Seattle in Feb 2010 my PET showed that jump…so I went back to Mayo and my surgeon had agreed and did the thoracotomy,,,,dang that was an awful awful surgery. He removed a melanoma from the lymph node and it was 6.8 at the biggest part.
But I was surgically made NED….Seattle said I no longer qualified for their trial because I had no measurable disease.
No trial was available for people NED…
Then a miracle happened and Dr Weber had one with MDX 1106 and peptides. I did 12 weeks of MDX 1106 ( anti PD 1 ) and peptides and remain NED and I continue every 3 months to receive the booster of MDX 1106. I get the booster for at least 2 years. The reason we did the trial was every doctor including Dr. W said i had a 99% chance of it coming back…considering the size and the location from the original site…I was staged with Metastatic Melanoma stage 4.
Science behind is the less tumor burden ( or in my case NED) the better chance we have of keeping it down. Dr W says if I remain NED for the 2 years of boosters it would be in best interest to try and remain on it forever…makes sense to me! On July 26 it will be 16 months NED.
-
- July 21, 2011 at 12:48 pm
Lynn,Congrats!!!
Been NED since surgery!!!!!
Why did you do radiation, temodar, surgery and vaccine trial of peptides and anti pd 1. since surgery? Do them as Adjuvant Therapy? You must have found all the trials that would accept NED patients. Please explain.
Warm regards
Jimmy B
-
- July 21, 2011 at 4:06 am
I keep thinking they intentionally leave surgery and radiation out of the picture and yet surgery is a preferred initial treatment for many people…Stage 4 NED since March 26, 2010. ..and had radiation, temodar, surgery and vaccine trial of peptides and anti pd 1. Been NED since surgery.
-
- July 21, 2011 at 12:55 am
Jim, I'll try again. the first couple of inches of response disappeared from here last try.
I find this data very interesting. Some of it I have been wondering what the numbers were.
It does need to be pointed out that these percentages are not really equal between different treatments, especially for their individual use.
The IL-2 numbers I have seen apply across the board to all type of melanoma mutations lumped together. Part of the Ipi trials were broad based, part of them were with restrictive criteria. The PLX4032 trials were very restrictive in the criteria as to which patients were allowed in their trials and they only allowed one of the BRAF mutatiions.
i would love to see more recent data that addresses the different oncoproteins and DNA mutations. I have not seen any results yet from any of the C-Kit trials. I have been "lucky" that my oncology Team listened to my request to try the C-kit drug, Gleevec, before it was in any formal clinical trials.. Without this targeted drug, which can only help a small percentage of melanoma patients, I would likely be dead. I suspect that having had IL-2 first may also be a factor in my survival to date. I have been in ontact with others that had the Gleevec alone for C-kit that had further progression after a period of remission.
As you know none of this is meant to knock your work in any way. I do send part of your work on to my Oncological team and do remember Dr Slingluff responding with favorable comment to you on some of what I forwarded.
UVA is working to get approval to greatly increase its presence in clinical trials. I keep telling them that we need more of your work in trials.
What is the longest term response you have seen reported for PLX4032 alone?
-
- July 21, 2011 at 12:03 pm
Jerry, I am not offended at all. The reason I didnot include C-kit is that it is a rare mutation and I don't have any offical response rate for you.
As for BRAF a mutation that effects 50% of melanoma patients, PLX 4032 has only been in trials for about 5 years. I heard that the longest living patient that was given PLX 4032 is about 3 yrs. Relapses occur because the cancer finds its way around the blocked BRAF pathway. That is why they started doing the combo of BRAF + MEK.
I been in touch with a patient that has done the BRAF + MEK therapy and is NED so far for about 6 months. Only time will tell.
I wish you all the best
Jimmy B
-
- July 22, 2011 at 1:10 am
Jimmy, I find it i nteresting that some people turn down the PLX-4032 because it has around a 50% chance of stopping working within a year. I am interested in how much longer people should also be looking at, rather than assuming that they will be the one to stop within the year. Even a year delay in progression might mean a lot in todays world. The addition of other treatments to it does indeed have promise. Have you seen any figures about whether it starts working on all BRAF V600E mutation patients or just some percentage? Yea, so many questions, haven't seen answers to all I want to know in what data I have run across. Wish I could get good high speed internet where I live!
Thanks again for all your research.
-
- July 22, 2011 at 1:10 am
Jimmy, I find it i nteresting that some people turn down the PLX-4032 because it has around a 50% chance of stopping working within a year. I am interested in how much longer people should also be looking at, rather than assuming that they will be the one to stop within the year. Even a year delay in progression might mean a lot in todays world. The addition of other treatments to it does indeed have promise. Have you seen any figures about whether it starts working on all BRAF V600E mutation patients or just some percentage? Yea, so many questions, haven't seen answers to all I want to know in what data I have run across. Wish I could get good high speed internet where I live!
Thanks again for all your research.
-
- July 21, 2011 at 12:03 pm
Jerry, I am not offended at all. The reason I didnot include C-kit is that it is a rare mutation and I don't have any offical response rate for you.
As for BRAF a mutation that effects 50% of melanoma patients, PLX 4032 has only been in trials for about 5 years. I heard that the longest living patient that was given PLX 4032 is about 3 yrs. Relapses occur because the cancer finds its way around the blocked BRAF pathway. That is why they started doing the combo of BRAF + MEK.
I been in touch with a patient that has done the BRAF + MEK therapy and is NED so far for about 6 months. Only time will tell.
I wish you all the best
Jimmy B
-
- July 21, 2011 at 12:55 am
Jim, I'll try again. the first couple of inches of response disappeared from here last try.
I find this data very interesting. Some of it I have been wondering what the numbers were.
It does need to be pointed out that these percentages are not really equal between different treatments, especially for their individual use.
The IL-2 numbers I have seen apply across the board to all type of melanoma mutations lumped together. Part of the Ipi trials were broad based, part of them were with restrictive criteria. The PLX4032 trials were very restrictive in the criteria as to which patients were allowed in their trials and they only allowed one of the BRAF mutatiions.
i would love to see more recent data that addresses the different oncoproteins and DNA mutations. I have not seen any results yet from any of the C-Kit trials. I have been "lucky" that my oncology Team listened to my request to try the C-kit drug, Gleevec, before it was in any formal clinical trials.. Without this targeted drug, which can only help a small percentage of melanoma patients, I would likely be dead. I suspect that having had IL-2 first may also be a factor in my survival to date. I have been in ontact with others that had the Gleevec alone for C-kit that had further progression after a period of remission.
As you know none of this is meant to knock your work in any way. I do send part of your work on to my Oncological team and do remember Dr Slingluff responding with favorable comment to you on some of what I forwarded.
UVA is working to get approval to greatly increase its presence in clinical trials. I keep telling them that we need more of your work in trials.
What is the longest term response you have seen reported for PLX4032 alone?
-
- July 21, 2011 at 2:17 am
Jim —
As a Stage 3 patient with an initial bulky MUP tumor and subsequent soft tissue localized recurrence, I am looking with real interest at all Stage 4 data. I wonder if you are familiar with any Stage 3 (adjuvant or neo-adjuvant) data on time to progression or whatever else one might measure.
Thanks for all your good work.
-
- July 21, 2011 at 12:17 pm
Time to progression is hard to get your arms around. Every patient is different. For myself, My cancer was Nodular Melanoma, the very aggressive kind. Progression was about a month.
I have a friend that did surgery, and his melanoma did not come back for 10 years.
If I was starting all over again, I would be looking for a diagnostic test for micro- Metastasis. Like TA90
TA90 is a 90-kd tumor-associated antigen that is expressed by >70% of melanomas. After curative resection of malignant melanoma, patients with occult metastasis may exhibit elevated levels of a TA90-IgG immune complex (TA90-IC).1 Several reports have indicated that TA90-IC is a sensitive and specific marker of recurrence in patients with malignant melanoma and is associated with shortened survival.1-4 Patients with TA90-IC detected early after curative resection of American Joint Committee on Cancer (AJCC) Stage I to III melanoma were found to have significantly lower 5-year overall survival (36% vs 84%, P <.001) and disease-free survival (24% vs 74%, P <.001) than TA90-IC-negative patients.2 In that study, TA90-IC status was independent of standard prognostic factors, including lymph-node status; Breslow depth was the only other significant predictor of outcome in multivariate analysis. Similar results were found for patients with thick (≥4 mm) melanomas.3
Serial monitoring of TA90-IC levels after curative resection of Stage I to III melanoma may also help predict recurrence, even in patients who are initially TA90-IC negative. Kelley et al found that 58 of 74 (78%) patients who developed distant metastasis had at least 2 consecutive positive TA90-IC results, first elevated an average of 19 months before clinical evidence of recurrence.2 Only 20 of 88 (23%) patients without metastasis had at least 2 positive TA90-IC results; when patients who received a polyvalent melanoma vaccine (known to elicit TA90-IC formation) were excluded, the sensitivity (92%) and specificity (86%) of the assay increased. Thus, detection of TA90-IC might be helpful in selecting patients for early intervention, when adjuvant therapy may be most effective.
Source:
http://www.questdiagnostics.com/hcp/intguide/jsp/showintguidepage.jsp?fn=TS_TA90.htm
Warm regards
Jimmy B
-
- July 21, 2011 at 12:17 pm
Time to progression is hard to get your arms around. Every patient is different. For myself, My cancer was Nodular Melanoma, the very aggressive kind. Progression was about a month.
I have a friend that did surgery, and his melanoma did not come back for 10 years.
If I was starting all over again, I would be looking for a diagnostic test for micro- Metastasis. Like TA90
TA90 is a 90-kd tumor-associated antigen that is expressed by >70% of melanomas. After curative resection of malignant melanoma, patients with occult metastasis may exhibit elevated levels of a TA90-IgG immune complex (TA90-IC).1 Several reports have indicated that TA90-IC is a sensitive and specific marker of recurrence in patients with malignant melanoma and is associated with shortened survival.1-4 Patients with TA90-IC detected early after curative resection of American Joint Committee on Cancer (AJCC) Stage I to III melanoma were found to have significantly lower 5-year overall survival (36% vs 84%, P <.001) and disease-free survival (24% vs 74%, P <.001) than TA90-IC-negative patients.2 In that study, TA90-IC status was independent of standard prognostic factors, including lymph-node status; Breslow depth was the only other significant predictor of outcome in multivariate analysis. Similar results were found for patients with thick (≥4 mm) melanomas.3
Serial monitoring of TA90-IC levels after curative resection of Stage I to III melanoma may also help predict recurrence, even in patients who are initially TA90-IC negative. Kelley et al found that 58 of 74 (78%) patients who developed distant metastasis had at least 2 consecutive positive TA90-IC results, first elevated an average of 19 months before clinical evidence of recurrence.2 Only 20 of 88 (23%) patients without metastasis had at least 2 positive TA90-IC results; when patients who received a polyvalent melanoma vaccine (known to elicit TA90-IC formation) were excluded, the sensitivity (92%) and specificity (86%) of the assay increased. Thus, detection of TA90-IC might be helpful in selecting patients for early intervention, when adjuvant therapy may be most effective.
Source:
http://www.questdiagnostics.com/hcp/intguide/jsp/showintguidepage.jsp?fn=TS_TA90.htm
Warm regards
Jimmy B
-
- July 21, 2011 at 2:17 am
Jim —
As a Stage 3 patient with an initial bulky MUP tumor and subsequent soft tissue localized recurrence, I am looking with real interest at all Stage 4 data. I wonder if you are familiar with any Stage 3 (adjuvant or neo-adjuvant) data on time to progression or whatever else one might measure.
Thanks for all your good work.
-
- July 21, 2011 at 11:10 am
Thank you, as always Jim, for putting the numbers in a way we can all understand. Your knowledge is an asset to this board. I do not understand why the doctors will not combine therapies – even giving them in succession if not at the same time. What is your opinion on a combination B-RAF and IPI for those who are positive for the mutation?
-
- July 21, 2011 at 12:41 pm
Maria,
Combination B-RAF and IPI is coming. When, 6- 12 months if all parties come together.
I would like to see B-RAF + MEK + IPI .
In my research, I was trying to find out how the dying tumors cells die. Ethier by Necrosis or Apoptosis.
It makes a difference because if is Apoptosis, or programmed cell death, no Danger Signals ( Alarmins) are generated and without Danger Signals , no Immune response.

Apoptosis, or programmed cell death, is a normal component of the development and health of multicellular organisms. Cells die in response to a variety of stimuli and during apoptosis they do so in a controlled, regulated fashion. This makes apoptosis distinct from another form of cell death called necrosis in which uncontrolled cell death leads to lysis of cells, inflammatory responses and, potentially, to serious health problems. Apoptosis, by contrast, is a process in which cells play an active role in their own death (which is why apoptosis is often referred to as cell suicide).
-
- July 21, 2011 at 12:41 pm
Maria,
Combination B-RAF and IPI is coming. When, 6- 12 months if all parties come together.
I would like to see B-RAF + MEK + IPI .
In my research, I was trying to find out how the dying tumors cells die. Ethier by Necrosis or Apoptosis.
It makes a difference because if is Apoptosis, or programmed cell death, no Danger Signals ( Alarmins) are generated and without Danger Signals , no Immune response.

Apoptosis, or programmed cell death, is a normal component of the development and health of multicellular organisms. Cells die in response to a variety of stimuli and during apoptosis they do so in a controlled, regulated fashion. This makes apoptosis distinct from another form of cell death called necrosis in which uncontrolled cell death leads to lysis of cells, inflammatory responses and, potentially, to serious health problems. Apoptosis, by contrast, is a process in which cells play an active role in their own death (which is why apoptosis is often referred to as cell suicide).
-
- July 22, 2011 at 5:10 am
Maria, a short answer for why doctors do not give patients combinations of Ipi and B-RAFis simply our system of medicine. No B-Raf drug has been approved by the FDA. It cannot be used in conjunction with other things at present. It can only be used in Clinical approved trials Ipi and other things can now be tried by doctors since it is an FDA approved treatment. When Jimmy talks about things coming together for a trial, this will involve at least two different companies, the government and medical centers. This type trial is something that Tim, our Director has been workiing on gettiing starterd.
It is imperative that it is approved for this type combinational trial to be provided in the future. Personally, it would appear feasable that after Phase one clinical trials have set viable dosages for patients of individual treatments that they could be looked at harder for combinational trials. They are also worried about lawsuits. They are afraid of being sued if they do anything that will harm "a dying person!". Excuse me if I get very irritated with this atitude. (we have too many lawyers in Congress!). Acertain amount of caution is necessary, but to what extreme?
-
- July 22, 2011 at 5:10 am
Maria, a short answer for why doctors do not give patients combinations of Ipi and B-RAFis simply our system of medicine. No B-Raf drug has been approved by the FDA. It cannot be used in conjunction with other things at present. It can only be used in Clinical approved trials Ipi and other things can now be tried by doctors since it is an FDA approved treatment. When Jimmy talks about things coming together for a trial, this will involve at least two different companies, the government and medical centers. This type trial is something that Tim, our Director has been workiing on gettiing starterd.
It is imperative that it is approved for this type combinational trial to be provided in the future. Personally, it would appear feasable that after Phase one clinical trials have set viable dosages for patients of individual treatments that they could be looked at harder for combinational trials. They are also worried about lawsuits. They are afraid of being sued if they do anything that will harm "a dying person!". Excuse me if I get very irritated with this atitude. (we have too many lawyers in Congress!). Acertain amount of caution is necessary, but to what extreme?
-
- July 22, 2011 at 11:43 am
Thanks Jerry! I wasn't familiar with the clinical trial process as it applies to combining therapies. I was under the impression that PLX-4032 was going to be approved by the end of July, but have not heard anything (a friend is on this drug and responding well). There was so much talk of combining them at ASCO, I just wish they would get the ball rolling for approval. I feel like lighting a fire under their arse and yelling – "what are you waiting for"? Maybe that will get their attention?!
-
- July 22, 2011 at 11:43 am
Thanks Jerry! I wasn't familiar with the clinical trial process as it applies to combining therapies. I was under the impression that PLX-4032 was going to be approved by the end of July, but have not heard anything (a friend is on this drug and responding well). There was so much talk of combining them at ASCO, I just wish they would get the ball rolling for approval. I feel like lighting a fire under their arse and yelling – "what are you waiting for"? Maybe that will get their attention?!
-
- July 21, 2011 at 11:10 am
Thank you, as always Jim, for putting the numbers in a way we can all understand. Your knowledge is an asset to this board. I do not understand why the doctors will not combine therapies – even giving them in succession if not at the same time. What is your opinion on a combination B-RAF and IPI for those who are positive for the mutation?
-
- July 22, 2011 at 11:54 am
thank you for your research and information. The timing of your information for me is really great as I find out next week if my tumor tests positive for Braf. In our talk withthe doctor of options I had asked if it was possible for me to do Braf and ipi at same time, doc said it wasnt done yet and worried what it might do to a person, but I could tell he also wondered about that combo. . I was told what they would do is do for me is Braf (if positive for it) and then as soon as it looked like things were not working jump to ipi. I am in some pain so hoping for some relief and they thought Braf would help me best with that.
I will have to research MEK I didnt hear about that at all. Operating is out for me as they said my melanoma is traveling and they want to get a hold on that.
sorry I am not the brightest bulb at understanding any of this – so please excuse my ignorance – I do appreciate all your research it helps alot for people like me who struggle to wrap their head around all that is out there. Thank you 🙂
laurie from maine
-
- July 22, 2011 at 11:54 am
thank you for your research and information. The timing of your information for me is really great as I find out next week if my tumor tests positive for Braf. In our talk withthe doctor of options I had asked if it was possible for me to do Braf and ipi at same time, doc said it wasnt done yet and worried what it might do to a person, but I could tell he also wondered about that combo. . I was told what they would do is do for me is Braf (if positive for it) and then as soon as it looked like things were not working jump to ipi. I am in some pain so hoping for some relief and they thought Braf would help me best with that.
I will have to research MEK I didnt hear about that at all. Operating is out for me as they said my melanoma is traveling and they want to get a hold on that.
sorry I am not the brightest bulb at understanding any of this – so please excuse my ignorance – I do appreciate all your research it helps alot for people like me who struggle to wrap their head around all that is out there. Thank you 🙂
laurie from maine
-
- July 23, 2011 at 12:01 pm
Laurie,
Just for the record, it is not ignorance. You are here on this board because you want to surround yourself with others who have walked the same road as you – and trust me, none of them were experts on mel prior to being diagnosed. You searching for info and treatment options is a sign of intelligence. If you have questions (as silly as they may seem), ask them. Knowledge is power, and it can go along way in your battle with this beast. There are alot of informed people (and NED) on this board regarding Stage IV – and it really should give you hope.
I wish you all the best,
Maria
P.S. – Jimmy B posted that a combination therapy is in the pipeline. Stay positive!
-
- July 23, 2011 at 12:01 pm
Laurie,
Just for the record, it is not ignorance. You are here on this board because you want to surround yourself with others who have walked the same road as you – and trust me, none of them were experts on mel prior to being diagnosed. You searching for info and treatment options is a sign of intelligence. If you have questions (as silly as they may seem), ask them. Knowledge is power, and it can go along way in your battle with this beast. There are alot of informed people (and NED) on this board regarding Stage IV – and it really should give you hope.
I wish you all the best,
Maria
P.S. – Jimmy B posted that a combination therapy is in the pipeline. Stay positive!
-
- You must be logged in to reply to this topic.

