› Forums › General Melanoma Community › The Anti-PD-1 Therapy Race for Approval by the FDA is On
- This topic has 29 replies, 9 voices, and was last updated 14 years, 4 months ago by
benp.
- Post
-
- September 28, 2011 at 2:36 pm
When it rains, it pours!!!! Melanoma patients over the last twenty years have not seen any progress in the fight to cure Melanoma. That has all changed in 2011 when the FDA approved Yervoy (Ipi..Ipilimumab), an anti-CTLA-4 monoclonal antibody and Zelboraf (vemurafenib). Well, this all going to change Melanoma from a cancer to a chronic disease that may be stabilized or even cured. The new Kid on the block( PD-1) is another Surface molecule that is unregulated when the T-cells are activated.
When it rains, it pours!!!! Melanoma patients over the last twenty years have not seen any progress in the fight to cure Melanoma. That has all changed in 2011 when the FDA approved Yervoy (Ipi..Ipilimumab), an anti-CTLA-4 monoclonal antibody and Zelboraf (vemurafenib). Well, this all going to change Melanoma from a cancer to a chronic disease that may be stabilized or even cured. The new Kid on the block( PD-1) is another Surface molecule that is unregulated when the T-cells are activated. This molecule is time dependent , which means that over time it migrates to the surface. Base on the research today, PD-1 molecule causes global inhibition to activated T-cells and down regulates the IL-2 expression by the PI3K/Akt pathway. It also inhibits the ICOS molecule that is an important co-stimulator for the T-cells.
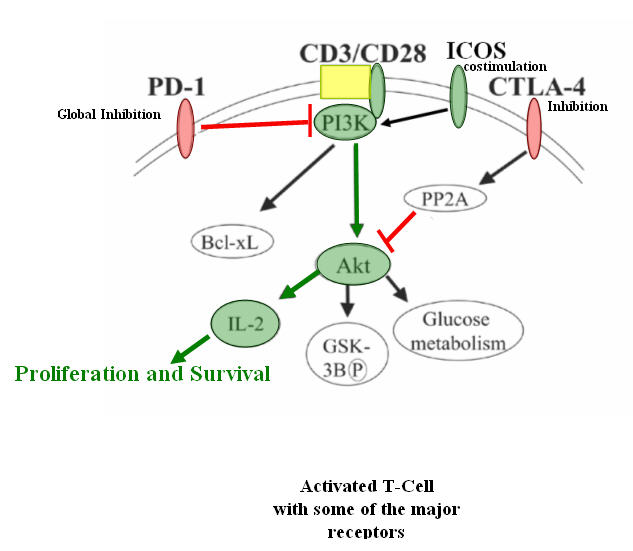
So with stakes high to be the first to market, Bristol Myer Squibb, a little known company, Amplimmune, co-sponsored by GlaxoSmithKline and Curetech, a subsidiary of Teva Pharma of Israel are in the race of their lives. Winner takes all. And to throw Icing on the cake, the blockage of both inhibitors (PD-1 and CTLA-4) have shown remarkable ability to eradicate Melanoma tumors in mice.
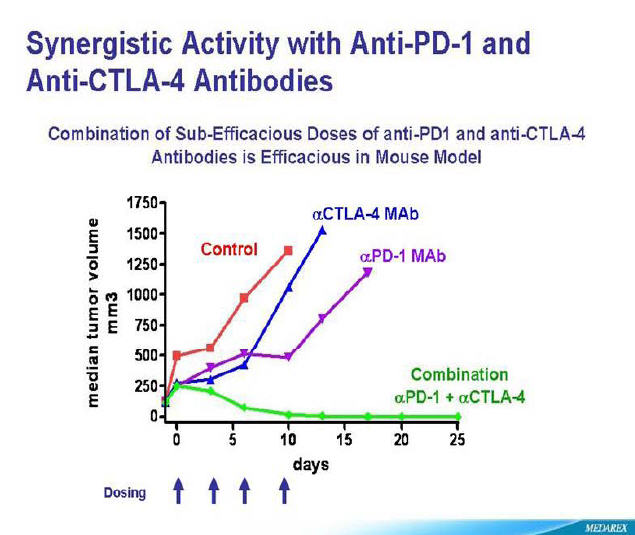
Bristol Myer Squibb seems to be leading this race with a clinical trials recruiting at Sloan Kettering in New York and Yale in Connecticut.
The study is “Dose-escalation Study of Combination BMS-936558 (MDX-1106) and Ipilimumab in Subjects With Unresectable Stage III or Stage IV Malignant Melanoma”
Trial: NCT01024231So with this in mind, if was seeking to try a clinical trial at time, I would seek out the combination first, then PD-1 and if all else fails, Anti-CTLA-4 therapy followed by IL-2.
I see a Stabilization/Cure on the horizon for this disease and others based on these immunotherapies.
“It is not the strongest of the species that survives, nor the most intelligent, but the one most responsive to change.”
~Charles Darwin~Take Care,
Jimmy B
- Replies
-
-
- September 28, 2011 at 3:12 pm
Like I've said the last few months that I've had this, I don't care what they come up with, I'll take it, if it keeps me around this earth longer! This is great news though. I'm not BRAF positive, so anything that is for ALL PATIENTS I absolutely love!
-
- September 28, 2011 at 3:12 pm
Like I've said the last few months that I've had this, I don't care what they come up with, I'll take it, if it keeps me around this earth longer! This is great news though. I'm not BRAF positive, so anything that is for ALL PATIENTS I absolutely love!
-
- September 28, 2011 at 3:14 pm
Hi Jimmy and everyone
I am a newbie & need recommendations.
I have mutliple sub q & multiple lung mets.
Would you recommend doing a inhibitor treatment (like the braf and/or mek ,the combo) then pd1,to first stablizie the tumor burden OR justdo PD1 first.
Thanks
A.
-
- September 28, 2011 at 3:14 pm
Hi Jimmy and everyone
I am a newbie & need recommendations.
I have mutliple sub q & multiple lung mets.
Would you recommend doing a inhibitor treatment (like the braf and/or mek ,the combo) then pd1,to first stablizie the tumor burden OR justdo PD1 first.
Thanks
A.
-
- September 28, 2011 at 3:54 pm
I would follow your Oncologist's recommendation.
To do the Braf + MEK therapy your tumor must be BRAF positive.
If you have high tumor burden and are BRAF +, I would ask your Onc. about the BRAF + MEK therapy.
If you have High tumor burden and BRAF-, I would seek out the combo Anti-PD-1 + Yervoy at Yale or Sloan Kettering.
If you have Low tumor burden and BRAF-, I would seek out Anti-PD-1 or Yervoy or HD IL-2.
Remember that this is a game of Chess, you want to have a plan action with Alternatives.
"The game is to win"
I hope this helps.
best regards,
Jimmy B

-
- September 28, 2011 at 3:54 pm
I would follow your Oncologist's recommendation.
To do the Braf + MEK therapy your tumor must be BRAF positive.
If you have high tumor burden and are BRAF +, I would ask your Onc. about the BRAF + MEK therapy.
If you have High tumor burden and BRAF-, I would seek out the combo Anti-PD-1 + Yervoy at Yale or Sloan Kettering.
If you have Low tumor burden and BRAF-, I would seek out Anti-PD-1 or Yervoy or HD IL-2.
Remember that this is a game of Chess, you want to have a plan action with Alternatives.
"The game is to win"
I hope this helps.
best regards,
Jimmy B

-
- September 28, 2011 at 4:34 pm
Thanks Jim,
I think this still has a few hoops to jump through, but based on the clinical data, it appears more promising and has less side effects than Yervoy.
-
- September 28, 2011 at 5:28 pm
i tried to get into these anti pd studies but they look for certain HLA blood types and i aint got it…which is frustrating…i also tried to get into the braf + mek trial and there were all kinds of delays, red tape so didn't get into that one…one cannot wait if they need treatment so i got on zelboraf…i got so frustrated with it all
boots
-
- September 28, 2011 at 5:28 pm
i tried to get into these anti pd studies but they look for certain HLA blood types and i aint got it…which is frustrating…i also tried to get into the braf + mek trial and there were all kinds of delays, red tape so didn't get into that one…one cannot wait if they need treatment so i got on zelboraf…i got so frustrated with it all
boots
-
- September 28, 2011 at 6:01 pm
I too have been looking into trials for anti-PD1 as a back-up plan for my father who is currently on Yervoy. It seems like the trials that out there right now will not take people who have previously used Yervoy, not sure why. Is there anyone out there who is in the anti-PD1 trial confirm this?
Thanks,
Chau
-
- September 28, 2011 at 6:01 pm
I too have been looking into trials for anti-PD1 as a back-up plan for my father who is currently on Yervoy. It seems like the trials that out there right now will not take people who have previously used Yervoy, not sure why. Is there anyone out there who is in the anti-PD1 trial confirm this?
Thanks,
Chau
-
- September 28, 2011 at 6:09 pm
Boots,
Did you try NCT01352884
This is a Phase 1, open-label, multi-center, first time in human study of AMP-224 in adult patients with cancer that is not responding to standard therapy. This study will be conducted in two stages consisting of a Dose-Escalation stage and an Expansion Stage.
AMP-224 is a Anti-PD-1 antibody.
CriteriaInclusion Criteria:
- Must be able to provide informed consent
- Must have solid tumor malignancy or cutaneous T-cell lymphoma that has relapsed and is refractory to standard therapy, or for which no standard therapy exists
- Must have measurable disease
- Must by at least 18 years old
- Must have adequate organ function
- Prior cancer therapies must have completed at least 4 weeks prior to first dose of AMP-224
Exclusion Criteria:
- Patients with follicular lymphoma
- Received >5 prior chemotherapeutic regimens (includes adjuvant therapy)
- Prior treatment with an anti-PD1 antibody therapy
- Known antibody response against prior antibody therapy or fusion protein therapeutics
- Major surgery within 4 weeks or radiotherapy within 2 weeks prior to first dose of AMP-224
- Prior allogeneic or autologous bone marrow or organ transplantation
- Known and/or a history or evidence of autoimmune disease
- Received an immunomodulatory drug within 2 weeks of first dose of AMP-224
- Active infections requiring antibiotics, physician monitoring, or recurrent fevers >100.4 degrees fahrenheit associated with a clinical diagnosis of active infection
- Patients with cirrhosis
- Clinically significant cardiac or electrocardiogram abnormalities
- History or evidence of HIV
- Active viral disease (except when the viral infection is associated with the malignancy)
- Pregnant or breastfeeding women
Best regards,
Jimmy B
-
- September 28, 2011 at 6:09 pm
Boots,
Did you try NCT01352884
This is a Phase 1, open-label, multi-center, first time in human study of AMP-224 in adult patients with cancer that is not responding to standard therapy. This study will be conducted in two stages consisting of a Dose-Escalation stage and an Expansion Stage.
AMP-224 is a Anti-PD-1 antibody.
CriteriaInclusion Criteria:
- Must be able to provide informed consent
- Must have solid tumor malignancy or cutaneous T-cell lymphoma that has relapsed and is refractory to standard therapy, or for which no standard therapy exists
- Must have measurable disease
- Must by at least 18 years old
- Must have adequate organ function
- Prior cancer therapies must have completed at least 4 weeks prior to first dose of AMP-224
Exclusion Criteria:
- Patients with follicular lymphoma
- Received >5 prior chemotherapeutic regimens (includes adjuvant therapy)
- Prior treatment with an anti-PD1 antibody therapy
- Known antibody response against prior antibody therapy or fusion protein therapeutics
- Major surgery within 4 weeks or radiotherapy within 2 weeks prior to first dose of AMP-224
- Prior allogeneic or autologous bone marrow or organ transplantation
- Known and/or a history or evidence of autoimmune disease
- Received an immunomodulatory drug within 2 weeks of first dose of AMP-224
- Active infections requiring antibiotics, physician monitoring, or recurrent fevers >100.4 degrees fahrenheit associated with a clinical diagnosis of active infection
- Patients with cirrhosis
- Clinically significant cardiac or electrocardiogram abnormalities
- History or evidence of HIV
- Active viral disease (except when the viral infection is associated with the malignancy)
- Pregnant or breastfeeding women
Best regards,
Jimmy B
-
- September 29, 2011 at 2:36 am
Boots, the hla testing is due to the vaccine component of a lot of trials, ie. pd1 with mdx1105. For single agent anti-pd1, the hla testing is not necessary according to my oncologist. The drug you are on seems to be showing a lot of promise for Braf positive folks. i would have gone that route but I'm nras, not braf. Hope you're feeking better. Robert
-
- September 29, 2011 at 2:36 am
Boots, the hla testing is due to the vaccine component of a lot of trials, ie. pd1 with mdx1105. For single agent anti-pd1, the hla testing is not necessary according to my oncologist. The drug you are on seems to be showing a lot of promise for Braf positive folks. i would have gone that route but I'm nras, not braf. Hope you're feeking better. Robert
-
- September 29, 2011 at 2:51 am
Yes…that is an important point to remember. I am in the Moffitt/Weber trial with anti-PD1 (MDX 1106) AND peptide vaccines in Tampa. That trial DOES require HLA typing due to the vaccine component…NOT the anti-PD1 component. For some good straight forward info on anti-PD1 check out- http://www.melanomaintl.org/ Click on the anti-PD1 webinar. You will be asked for your name and email address in order to watch, but that is all.
Good luck. C -
- September 29, 2011 at 2:51 am
Yes…that is an important point to remember. I am in the Moffitt/Weber trial with anti-PD1 (MDX 1106) AND peptide vaccines in Tampa. That trial DOES require HLA typing due to the vaccine component…NOT the anti-PD1 component. For some good straight forward info on anti-PD1 check out- http://www.melanomaintl.org/ Click on the anti-PD1 webinar. You will be asked for your name and email address in order to watch, but that is all.
Good luck. C
-
- October 9, 2011 at 5:50 pm
Jimmy B,
Am I reading this chart right regarding the combo of anti-PD1 and CTLA 4 that the tumor burden was reduced to 0 after 10 t0 15 days in the mouse model vs. 1250 to 1500 with pd1 alone? It looks amazing. I had scans on Thursday and the docs at UCSF want me to start a pk13 trial if I'm progressing or wait until their antiPD-1 trial starts in November if things are stable. Are you familiar with any studies of pk13 inhibitors in NRAS mutations? The Melanoma Molecular Model you posted shows PK13 inhibitor as the therepeutic approach to NRAS. Appreciate any comments or resources you may have.
Thanks you!
Robert
-
- October 9, 2011 at 5:50 pm
Jimmy B,
Am I reading this chart right regarding the combo of anti-PD1 and CTLA 4 that the tumor burden was reduced to 0 after 10 t0 15 days in the mouse model vs. 1250 to 1500 with pd1 alone? It looks amazing. I had scans on Thursday and the docs at UCSF want me to start a pk13 trial if I'm progressing or wait until their antiPD-1 trial starts in November if things are stable. Are you familiar with any studies of pk13 inhibitors in NRAS mutations? The Melanoma Molecular Model you posted shows PK13 inhibitor as the therepeutic approach to NRAS. Appreciate any comments or resources you may have.
Thanks you!
Robert
-
- October 9, 2011 at 5:50 pm
Jimmy B,
Am I reading this chart right regarding the combo of anti-PD1 and CTLA 4 that the tumor burden was reduced to 0 after 10 t0 15 days in the mouse model vs. 1250 to 1500 with pd1 alone? It looks amazing. I had scans on Thursday and the docs at UCSF want me to start a pk13 trial if I'm progressing or wait until their antiPD-1 trial starts in November if things are stable. Are you familiar with any studies of pk13 inhibitors in NRAS mutations? The Melanoma Molecular Model you posted shows PK13 inhibitor as the therepeutic approach to NRAS. Appreciate any comments or resources you may have.
Thanks you!
Robert
-
Tagged: cutaneous melanoma
- You must be logged in to reply to this topic.

