› Forums › General Melanoma Community › Identifying and overcoming immune barriers at the levelof the tumor microenvironment
- This topic has 9 replies, 2 voices, and was last updated 14 years, 4 months ago by
boot2aboot.
- Post
-
- October 14, 2011 at 2:27 am
Eleventh International Conference on Progress in Vaccination Against Cancer
10 – 13 October2011 Copenhagen, Denmark
Dr.Thomas Gajewski
University of Chicago, Chicago, IL, USA
Eleventh International Conference on Progress in Vaccination Against Cancer
10 – 13 October2011 Copenhagen, Denmark
Dr.Thomas Gajewski
University of Chicago, Chicago, IL, USA
Immunotherapeutic approaches for the treatment of melanoma, such as tumor antigen-based vaccines, can frequently boost immune responses. However, clinical responses as measured by tumor shrinkage are seen in only a minority of patients. This observation has prompted careful analysis of the tumor microenvironment for biologic correlates to clinical response and also to identify mechanisms of tumor resistance. Patients with advanced melanoma treated with antigen-specific vaccines had pre-treatment tumor biopsies analyzed by gene expression profiling. Supervised hierarchical clustering was performed based on clinical outcome. An expanded bank of tumors was analyzed to increase the sample size and better understand gene patterns.
Two major categories of melanoma metastases have been observed.
One subgroup of patient has an inflamed phenotype that includes expression of chemokines, T-cell markers, and other immunoregulatory factors. Clinical responders to melanoma vaccines appear to fall within this subset. This group also contains the highest expression of negative regulatory factors, including PD-L1, IDO, and FoxP3, suggesting that these immunosuppressive mechanisms may dominantly inhibit anti-tumor –cell function in those patients. In addition, absence of B7 expression supports classical T-cell anergy. Preclinical experiments have confirmed a critical role for these mechanisms in limiting anti-tumor T–cell efficacy in vivo, giving candidate treatment strategies for translation back into the clinic.
A second subset of patients is represented by tumors which are non-inflamed and lack chemokines for T cell recruitment. Therefore, a major barrier in these cases appears to be failed T–cell migration into tumor sites. Experimental strategies to augment T-cell migration can have important anti-tumor effects in preclinical models. The presence of the "inflamed" gene signature was associated with a type I IFN transcriptional profile, and murine experimental models have confirmed a critical role for type I IFN signaling in promoting adaptive immunity.
So one subset tumors has a suppresive nature that may be over riddden by Anti-CTLA-4 (Yervoy) and or Anti-PD-1 Therapy
The second subset is missing the "danger signal"
Cytokines are small proteins which allow cells of the immune system to communicate with one another via cytokine receptors expressed at the cell surface.
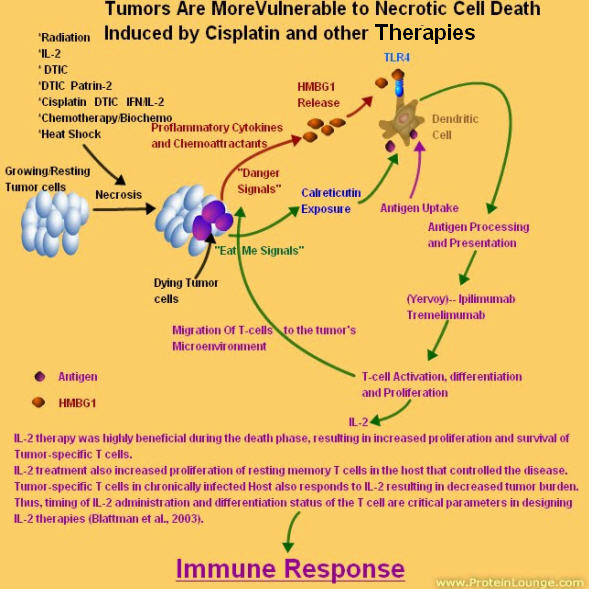
Activated macrophages defend against tumors by secreting cytokines to recruit secondary immune cells, presenting antigen to T cells, and by direct tumor cytotoxicity. Peritoneal macrophages harvested from melanoma-bearing mice are less cytotoxic to melanoma cells, and produce less superoxide, nitric oxide, and tumor necrosis factor-alpha (TNF-alpha) than those from nontumor-bearing mice. Similar impairment of macrophage activation occurs in vitro using media harvested from cultured melanoma cells.
Stimulation of Toll-like receptor 4 (TLR-4) activates macrophages and results in the release of TNF-alpha. It is hypothesized that melanoma inhibits macrophage activation by suppressing TLR-4 signaling.

http://2.bp.blogspot.com/-4TLHwjIx_XU/TpSCg80EcVI/AAAAAAAAAh4/j_EKCkplea4/s1600/Danger%2BSignal.jpg
Cytokines are small proteins which allow cells of the immune system to communicate with one another via cytokine receptors expressed at the cell surface.
Activated macrophages defend against tumors by secreting cytokines to recruit secondary immune cells, presenting antigen to T cells, and by direct tumor cytotoxicity. Peritoneal macrophages harvested from melanoma-bearing mice are less cytotoxic to melanoma cells, and produce less superoxide, nitric oxide, and tumor necrosis factor-alpha (TNF-alpha) than those from nontumor-bearing mice. Similar impairment of macrophage activation occurs in vitro using media harvested from cultured melanoma cells.
Activated Macrophages secrete the following cytokines under different conditions:
IL-1,IL-12,IL-6, IFN -gamma and TNF-alpha
So, if Melanoma suppresses Macrophage Activation, then the tumor microenviroment is missing IL-6 and other cytokines.
Interleukin 6 is a pro-inflammatory cytokine and is produced in response to infection and tissue injury. IL-6 exerts its effects on multiple cell types and can act systemically.
IL-6 stimulates liver secretion of acute phase proteins
IL-6 stimulates liver secretion of acute phase proteins
IL-6 stimulates B-lymphocytes to produce antibodies
IL-6 in concert with IL-1 causes T-cell activation
IL-6 induces STAT 3 Signaling
IL-6 Plus TGF-b induces the Th17 cell phenotype
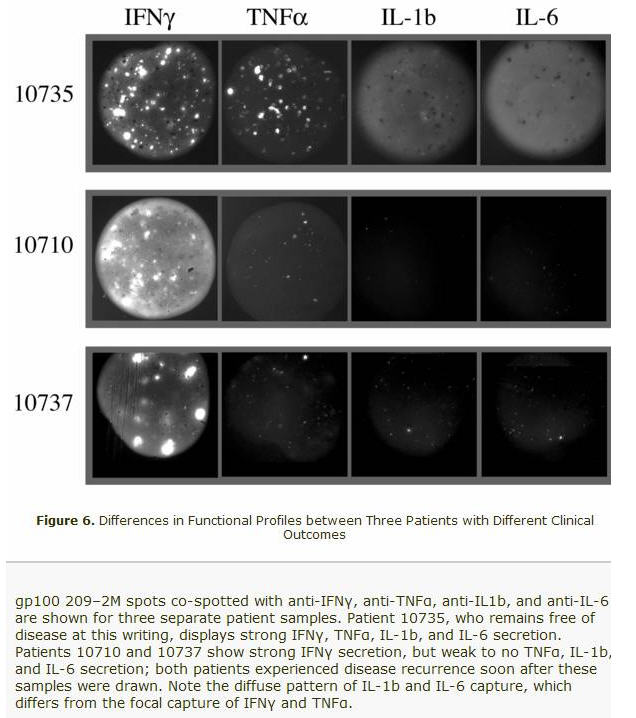
If you look at the above micrographs, you will see that the two patients that had Relapsed (10710 and 10737) had IL-1b and IL-6 missing. The Macrophages were not activated!!!! The "Danger Signal " known as inflammation was missing!
The missing combination of IL-1 and IL-6 meant no T-cell activation. And no induction of the Th17 phenotype. It is now becoming a lot more clearer based on Dr. Gajewski's findings.
- Replies
-
-
- October 14, 2011 at 2:36 am
It is hypothesized that melanoma inhibits macrophage activation by suppressing TLR-4 signaling.

-
- October 14, 2011 at 2:36 am
It is hypothesized that melanoma inhibits macrophage activation by suppressing TLR-4 signaling.


-
- October 14, 2011 at 2:36 am
It is hypothesized that melanoma inhibits macrophage activation by suppressing TLR-4 signaling.


-
- October 14, 2011 at 11:39 am
Based on Dr. Gajewski's finding on tumors, one must block the STAT3 signaling from the tumor. This will allow the activation of the (DCs) Dentritic Cells and the Macrophages.
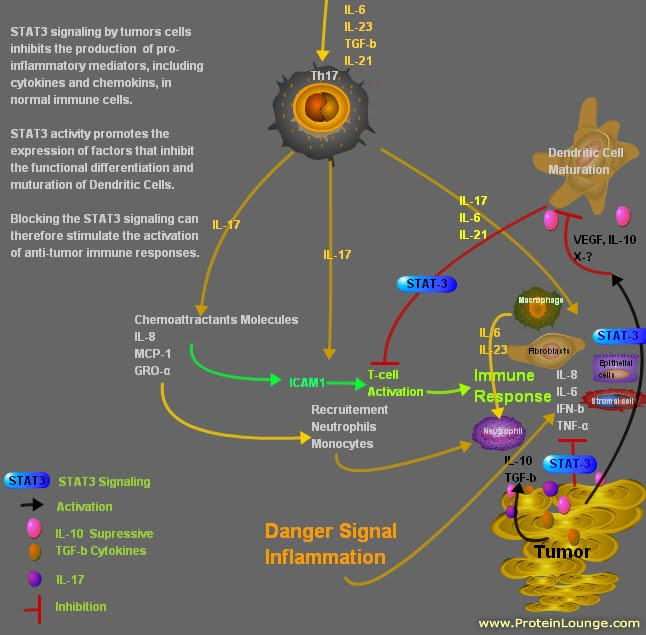
So in conclusion: There are two subtypes of tumors based on clinical and diagnostic testing.
1) tumors with high suppressive molecules like CTLA-4 and PD-1..etc with inflamed cytokines
2) tumors missing the inflamed cytokines, ckemokines and chemoattractants.
The first subtype can be treated with Anti-CTLA-4 (Yervoy) and Anti- PD-1 antibodies.
The second can be treated with STAT3 blocking agents along with Radiation, chemotherapy with DNA repair modulators
Now might be the time for a critical re-evaluation of our overall approaches to targeting STAT3 and for developing new models for disrupting the protein in order toaccomplish the goal of delivering clinically useful direct STAT3 inhibitors as novel anticanceragents in a timely manner.
Best regards
Jimmy B
-
- October 14, 2011 at 11:39 am
Based on Dr. Gajewski's finding on tumors, one must block the STAT3 signaling from the tumor. This will allow the activation of the (DCs) Dentritic Cells and the Macrophages.

So in conclusion: There are two subtypes of tumors based on clinical and diagnostic testing.
1) tumors with high suppressive molecules like CTLA-4 and PD-1..etc with inflamed cytokines
2) tumors missing the inflamed cytokines, ckemokines and chemoattractants.
The first subtype can be treated with Anti-CTLA-4 (Yervoy) and Anti- PD-1 antibodies.
The second can be treated with STAT3 blocking agents along with Radiation, chemotherapy with DNA repair modulators
Now might be the time for a critical re-evaluation of our overall approaches to targeting STAT3 and for developing new models for disrupting the protein in order toaccomplish the goal of delivering clinically useful direct STAT3 inhibitors as novel anticanceragents in a timely manner.
Best regards
Jimmy B
-
- October 17, 2011 at 6:01 pm
so Jimmy,i get the the first subset (ctl4 pathway) …don't understand 2nd subset…is 2nd subset be 'targeted' therapy like zelboraf that bridges broken Raf pathway? how does one determine other than trial and error what subset one is in? can it switch back and forth?
boots
-
- October 17, 2011 at 6:01 pm
so Jimmy,i get the the first subset (ctl4 pathway) …don't understand 2nd subset…is 2nd subset be 'targeted' therapy like zelboraf that bridges broken Raf pathway? how does one determine other than trial and error what subset one is in? can it switch back and forth?
boots
-
- October 17, 2011 at 6:01 pm
so Jimmy,i get the the first subset (ctl4 pathway) …don't understand 2nd subset…is 2nd subset be 'targeted' therapy like zelboraf that bridges broken Raf pathway? how does one determine other than trial and error what subset one is in? can it switch back and forth?
boots
-
- October 14, 2011 at 11:39 am
Based on Dr. Gajewski's finding on tumors, one must block the STAT3 signaling from the tumor. This will allow the activation of the (DCs) Dentritic Cells and the Macrophages.

So in conclusion: There are two subtypes of tumors based on clinical and diagnostic testing.
1) tumors with high suppressive molecules like CTLA-4 and PD-1..etc with inflamed cytokines
2) tumors missing the inflamed cytokines, ckemokines and chemoattractants.
The first subtype can be treated with Anti-CTLA-4 (Yervoy) and Anti- PD-1 antibodies.
The second can be treated with STAT3 blocking agents along with Radiation, chemotherapy with DNA repair modulators
Now might be the time for a critical re-evaluation of our overall approaches to targeting STAT3 and for developing new models for disrupting the protein in order toaccomplish the goal of delivering clinically useful direct STAT3 inhibitors as novel anticanceragents in a timely manner.
Best regards
Jimmy B
-
- You must be logged in to reply to this topic.

